Interconversion of hydrated protons at the interface between liquid water and platinum†
Abstract
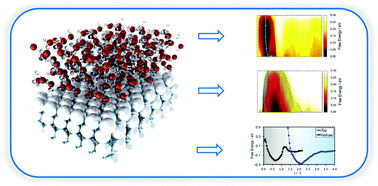
Hydrogen transfer is the fundamental step in electrochemistry involved in water splitting and the hydrogen evolution reaction (HER). However, the nature of this process at the solid–liquid interface has been little studied at the atomic level. In this work, we use ab initio molecular dynamics (AIMD) and umbrella sampling (US), giving us an accurate description of the dynamic processes associated with the solid–liquid environment. Based on this method, the free energy barriers were calculated at the H2O/Pt(111) interface, and a multistep mechanism has been proposed. We find that proton transfer is dictated by the strength of the solid–liquid interaction and the configuration of water molecules above the reaction site. In particular, we show that the surface adsorbed cations, which are confined to the interface above the top site position, act as vessels for enhanced hydrogen transfer to and from the surface. Our results could lead to significant mechanistic consequences for the HER, water splitting and solid–liquid reactions in general.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...