Ligand binding effects on the activation of the EGFR extracellular domain†
Abstract
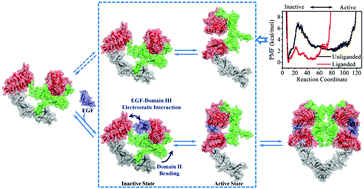
The epidermal growth factor receptor (EGFR) is one of the most common target proteins in anti-cancer therapy. The binding of the EGF ligand to the EGFR extracellular domain (EGFR-ECD) promotes its inactive-to-active conformational transition (activation) but the relevant detailed mechanism remains elusive still. Here, the structural characterization and energetics of the EGFR-ECD conformational transition with and without the binding of the EGF are quantitatively explored using an innovative enhanced sampling MD simulation method. Intriguingly, the EGF offers hydrophobic interactions (e.g., EGF residues of Tyr44 and Leu47) and electrostatic interactions (e.g., the EGF residues of Glu5, Asp11, Asp17, and Arg41) to play a dominant role in dragging domain III to close the ligand binding domain gap. Subsequently, the correlation between domains III and II is enhanced through salt-bridges among Glu376, Arg403, and Arg405 from domain III and Glu293, Glu295, and Arg300 from domain II. Finally, the structural bending of domain II is regulated to facilitate the disengagement of domain II from domain IV. In this regard, the functional conformational transition of EGFR-ECD is a consequence of the cooperative motion of protein domains driven by the EGF ligand binding. The present study shows a detailed scenario of the EGF induced activation of EGFR-ECD and provides valuable information for drug discovery targeting the EGFR.


 Please wait while we load your content...
Please wait while we load your content...