Electrochemical hydrogenation of non-aromatic carboxylic acid derivatives as a sustainable synthesis process: from catalyst design to device construction†
Abstract
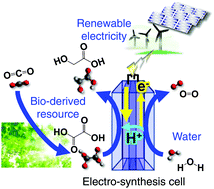
Electrochemical hydrogenation of a carboxylic acid using water as a hydrogen source is an environmentally friendly synthetic process for upgrading bio-based chemicals. We systematically studied electrochemical hydrogenation of non-aromatic carboxylic acid derivatives on anatase TiO2 by a combination of experimental analyses and density functional theory calculations, which for the first time shed light on mechanistic insights for the electrochemical hydrogenation of carboxylic acids. Development of a substrate permeable TiO2 cathode enabled construction of a flow-type electrolyser, i.e., a so-called polymer electrode alcohol synthesis cell (PEAEC) for the continuous synthesis of an alcoholic compound from a carboxylic acid. We demonstrated the highly efficient and selective conversion of oxalic acid to produce glycolic acid, which can be regarded as direct electric power storage into an easily treatable alcoholic compound.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...