Luminescence spectroscopy of oxazine dye cations isolated in vacuo†
Abstract
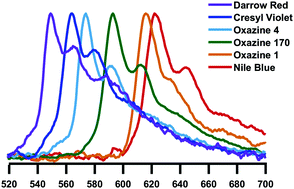
Here we report gas-phase action and luminescence spectra of cationic dyes derived from oxazine: cresyl violet (CV+), oxazine 170 (Ox-170+), nile blue (NB+), darrow red (DR+), oxazine 1 (Ox-1+), oxazine 4 (Ox-4+), and brilliant cresyl blue (BCB+). The first four have a benzofused structure, which results in asymmetric charge distributions along the long axis. The positive charge is also asymmetrically distributed in BCB+ while Ox-1+ and Ox-4+ are symmetric. As the ions are isolated in vacuo, there are no interactions with solvent molecules or counter ions, and the effect of chemical modifications is therefore more easily revealed than from solution-phase experiments. The transition energy decreases in the order: DR+ > CV+ > Ox-4+ > Ox-170+ > BCB+ > Ox-1+ > NB+, and the fluorescence from BCB+ is less than from the others. We discuss the results based on electron delocalisation, degree of charge-transfer character, rigidity of the chromophore structure, and substituents.


 Please wait while we load your content...
Please wait while we load your content...