Medium-sized rings: conformational preferences in cyclooctanone driven by transannular repulsive interactions†
Abstract
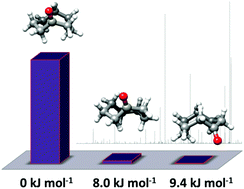
The conformational properties of medium-sized rings are of relevance to understand their intramolecular interactions and reactivity. Here we have characterised the conformational landscape of the eight-membered ketone cyclooctanone by broadband rotational spectroscopy in combination with quantum-chemistry calculations. Three conformers, two boat-chair and one twisted boat chair configurations, have been identified and their spectroscopic parameters determined. Cyclooctanone predominantly exists in the global minimum boat-chair conformation, whose bond lengths and angles have been determined for the first time. The relative abundance of the global minimum with respect to the second conformer in the energy ordering, a twisted boat-chair, is 40 : 1 in all carrier gases used in the supersonic expansion. The conformational preferences of cyclooctanone are driven by minimisation of repulsive non-bonded transannular interactions.


 Please wait while we load your content...
Please wait while we load your content...