Na+-binding modes involved in thrombin's allosteric response as revealed by molecular dynamics simulations, correlation networks and Markov modeling†
Abstract
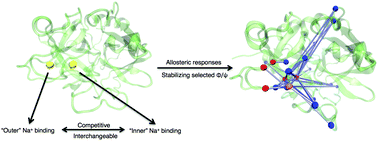
The monovalent sodium ion (Na+) is a critical modulator of thrombin. However, the mechanism of thrombin's activation by Na+ has been widely debated for more than twenty years. Details of the linkage between thrombin and Na+ remain vague due to limited temporal and spatial resolution in experiments. In this work, we combine microsecond scale atomic-detailed molecular dynamics simulations with correlation network analyses and hidden Markov modeling to probe the detailed thermodynamic and kinetic picture of Na+-binding events and their resulting allosteric responses in thrombin. We reveal that ASP189 and ALA190 comprise a stable Na+-binding site (referred as “inner” Na+-binding site) along with the previously known one (referred as “outer” Na+-binding site). The corresponding newly identified Na+-binding mode introduces significant allosteric responses in thrombin's regulatory regions by stabilizing selected torsion angles of residues responsive to Na+-binding. Our Markov model indicates that the bound Na+ prefers to transfer between the two Na+-binding sites when an unbinding event takes place. These results suggest a testable hypothesis of a substrate-driven Na+ migration (ΔG ∼ 1.7 kcal mol−1) from the “inner” Na+-binding site to the “outer” one during thrombin's catalytic activities. The binding of a Na+ ion at the “inner” Na+-binding site should be inferred as a prerequisite for thrombin's efficient recognition to the substrate, which opens a new angle for our understanding of Na+-binding's allosteric activation on thrombin and sheds light on detailed processes in thrombin's activation.


 Please wait while we load your content...
Please wait while we load your content...