Computational discovery and characterization of new B2O phases†
Abstract
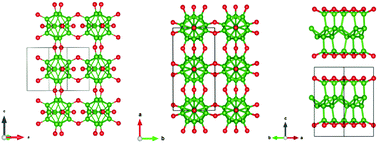
We present computational discoveries of new structural phases of the B2O compound exhibiting novel bonding networks and electronic states at ambient and elevated pressures. Our advanced crystal structure searches in conjunction with density functional theory calculations have identified an orthorhombic phase of B2O that is energetically stable at ambient pressure and contains an intriguing bonding network of icosahedral B12 clusters bridged by oxygen atoms. As pressure increases above 1.9 GPa, a structural transformation takes the orthorhombic B2O into a pseudo-layered trigonal phase. We have performed extensive studies to investigate the evolution of chemical bonds and electronic states associated with the B12 icosahedral unit in the orthorhombic phase and the covalent B–O bonds in the trigonal phase. We have also examined the nature of the charge carriers and their coupling to the lattice vibrations in the newly identified B2O crystals. Interestingly, our results indicate that both B2O phases become superconducting at low temperatures, with transition temperatures of 6.4 K and 5.9 K, respectively, in the ambient and high-pressure phase. The present findings establish new B2O phases and characterize their structural and electronic properties, which offer insights and guidance for exploration toward further fundamental understanding and potential synthesis and application.


 Please wait while we load your content...
Please wait while we load your content...