Thermodynamic limits of countercurrent reactor systems, with examples in membrane reactors and the ceria redox cycle
Abstract
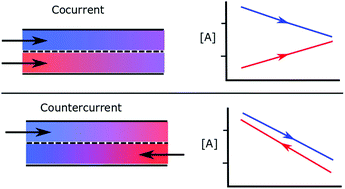
Countercurrent reactors can be utilized in chemical reaction systems which involve either a reaction between flows of different phases, or reactions between flows separated by a selective permeable membrane. This idea is quite similar in nature to a countercurrent heat exchanger, where the inlet of one participating flow is exposed to the outlet of the opposite flow. A countercurrent configuration can therefore improve the reaction conversion extent and transport properties. Here we formulate a straightforward approach in terms of an exchange coordinate, in order to determine an upper bound of species exchange in such systems, subject to the second law of thermodynamics and conservation of mass. The methodology is independent of the specifics of reactor design and can be generally applied to determine the maximum thermodynamic benefit of using a countercurrent reactor. We then demonstrate the analysis for a number of thermochemical fuel production routes; membrane thermolysis of carbon dioxide, dry methane reforming across a membrane, reverse water gas shift across a membrane, and the thermochemical ceria cycle.


 Please wait while we load your content...
Please wait while we load your content...