New insights into the dissociation dynamics of methylated anilines†
Abstract
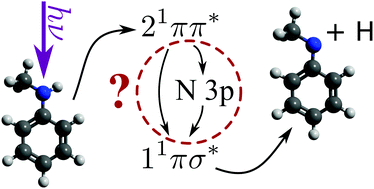
Aniline, an important model system for biological chromophores, undergoes ultrafast H-atom loss upon absorption of an ultraviolet photon. By varying the number and position of methyl substituents on both the aromatic ring and amine functional group, we explore the ultrafast production of photofragments as a function of molecular structure. Both N-methyl aniline and 3,5-dimethyl aniline show altered H-atom loss behaviour compared to aniline, while no evidence for CH3 loss was found from either N-methyl aniline or N,N-dimethyl aniline. With the addition of time-resolved photoelectron spectroscopy, the photofragment appearance times are matched to excited state relaxation pathways. Evidence for a sequential excited state relaxation mechanism, potentially involving a valence-to-Rydberg decay mechanism, will be presented. Such a global, bottom-up approach to molecular photochemistry is crucial to understanding the dissociative pathways and excited state decay mechanisms of biomolecule photoprotection in nature.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...