An ab initio study of sensing applications of MoB2 monolayer: a potential gas sensor†
Abstract
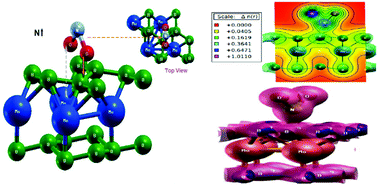
Using first principles density functional theory, we have studied the interaction mechanism of NO2 and SO2 gas molecules on an MoB2 monolayer, for gas sensing applications. The selectivity for a particular gas by the sensor has been analyzed through electronic structure calculations and adsorption studies. The calculations have been performed by considering the fact that the MoB2 monolayer as a sensing material encounters a change in its electrical properties, when gas molecules with different orientations get adsorbed on the surface. From the density of states study, we find better selectivity for NO2 as compared to SO2, as the latter leaves the electronic structure of the sensing material unaffected. Further, the adsorption curves support the above fact as the larger value of adsorption energy (Ead ∼ −1 eV) for NO2 indicates stronger adsorption. The chemisorptive nature for NO2, in contrast with the relatively weaker physisorption for SO2, additionally supports the fact that NO2 gas has a better perspective for MoB2 sensor application. Charge density plots for each case are in good agreement with the above conclusions. The faster recovery time attributes the MoB2 monolayer better as a sensor material for NO2 interaction.


 Please wait while we load your content...
Please wait while we load your content...