Electron-induced chemistry of surface-grown coordination polymers with different linker anions†
Abstract
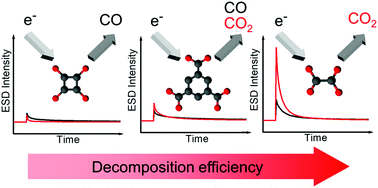
Electron beam processing of surface-grown coordination polymers is a versatile approach to the fabrication of nanoscale surface structures. Depending on their molecular components, these materials can be converted into pure metallic particles or they can be activated to become a template for the spatially selective decomposition of suitable gaseous precursor molecules and subsequent autocatalytic growth of deposits. However, insight into the fundamental electron-induced chemistry for such processes has been scarce so far. Therefore, we investigated the electron-induced reactions of three self-assembled copper-containing materials, namely, copper(II) oxalate, copper(II) squarate, and copper(II) 1,3,5-benzenetricarboxylate (HKUST-1) which were grown on the surface of self-assembled monolayers of mercaptoundecanoic acid in a layer-by-layer approach from copper(II) acetate and various linker molecules. Changes incurred to these materials during electron irradiation were monitored by four complementary techniques. Reflection absorption infrared spectroscopy (RAIRS) and X-ray photoelectron spectroscopy (XPS) were used to identify the chemical species that are formed upon electron exposure. The temporal evolution of electron-stimulated desorption (ESD) of neutral volatile fragments was monitored to reveal the kinetics governing the decomposition of the different materials. Furthermore, the morphology was investigated by helium ion microscopy (HIM). A detailed analysis of the results for the different linker molecules provides new insights into the electron-induced chemistry of such surface-grown layers.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...