Impact of morphology, side-chains, and crystallinity on charge-transport properties of π-extended double helicenes†
Abstract
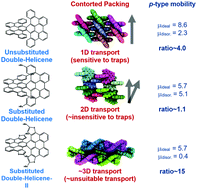
We report a computational study on the effect of side-chain substitution, heteroaromatic substitution and unique crystal packing on the charge transport and mobility of three double helicene molecules. These double helicene (DH) molecules, having curved π-conjugation, are structural hybrids of non-planar [6]helicene and planar tribenzo[b,n,pqr]perylene (TBP). We find that side-chain substitution has only a effect on intrinsic electronic properties in DHs but dramatically impacts the packing arrangement, morphologies and transport network, exhibited in calculated charge transport parameters. Interestingly, the dimensionality of the transport evolves from one dimensional to three dimensional with side-chain substitution (DH2) and heteroaromatic substitution (DH3). Using two different well-known transport models, we have established a direct link between the morphology, transport connectivity, and hole mobilities. While both unsubstituted and substituted DHs exhibit high hole mobilities in the ordered phase, the results show that with inclusion of positional disorder, the mobilities of disordered DH1 and DH3 are lower while the mobility of DH2 remain nearly unchanged. We relate this effect to the dimensionality of their unique transport networks. These DH molecules are promising organic semiconductors with high mobilities in ordered and disordered phases, with predicted values that lie in the range of ∼1 to 10 cm2 V−1 s−1.


 Please wait while we load your content...
Please wait while we load your content...