Time-resolved multirotational dynamics of single solution-phase tau proteins reveals details of conformational variation†
Abstract
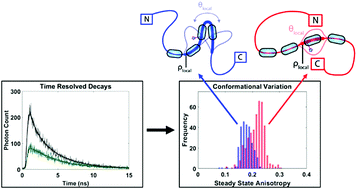
Intrinsically disordered proteins (IDPs) are crucial to many cellular processes and have been linked to neurodegenerative diseases. Single molecules of tau, an IDP associated with Alzheimer's disease, are trapped in solution using a microfluidic device, and a time-resolved fluorescence anisotropy decay is recorded for each molecule. Multiple rotational components are resolved and a novel k-means algorithm is used to sort the molecules into two families of conformations. Differences in rotational dynamics suggest a change in the rigidity and steric hindrance surrounding a sequence (306VQIVYK311) which is central to paired helical filament formation. This single-molecule approach can be applied to other IDPs to resolve heterogeneous populations and underlying differences in conformational dynamics.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...