First-principles investigation of the double ESIPT process in a thiophene-based dye†
Abstract
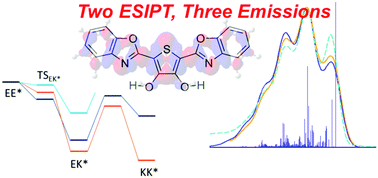
The unusual emission spectrum of 2,5-bis(benzoxazol-2-yl)thiophene-3,4-diol (BBTP) is investigated. The complexity of the emission spectrum of this dye is due to the presence of two excited-state intramolecular proton transfer (ESIPT) sites that give rise to three non-equivalent tautomers. The different maxima were experimentally attributed to the initial double enol form, the single ESIPT enol–keto tautomer, and the double ESIPT structure. Our simulations, based on Time Dependent Density Functional Theory (TD-DFT) and post Hartree–Fock methods [ADC(2) and CC2] coupled to different schemes to include the solvent polarisation response, are able to reproduce the key experimental outcomes. Moreover, we prove that for solving the inconsistencies present in earlier theoretical studies, a state-specific solvation approach is needed: one should go beyond the standard linear-response scheme in polarisable dielectric models. Finally, using a validated model, we explore the impact of substitution by donor and acceptor groups.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...