Disulfide bond photochemistry: the effects of higher excited states and different molecular geometries on disulfide bond cleavage†
Abstract
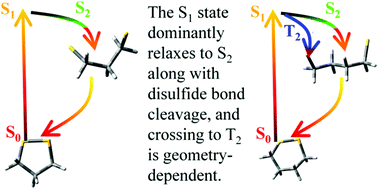
The disulfide bond is prone to ultraviolet light-induced cleavage, but the microscopic details of the light-activated bond breakage remain elusive. Here, we carry out quantum chemical calculations and the first TSH simulation of the excited state dynamics of disulfides at the MS-CASPT2 level. We demonstrate that during relaxation of the S1 state, IC to the S2 state is the predominant relaxation pathway and efficient ISC to the T2 state is geometry-dependent. Moreover, the bond cleavage leads to a strong coupling region of singlet–triplet quasidegeneracy and enlarged SOC, from which both returning to the S0 state and effective triplet formation happen. On the basis of the simulation results, the proposed electronic relaxation mechanism of light-activated disulfides is S1 → S2(T2) → region of singlet–triplet quasidegeneracy → S0, which emphasizes the competitive participation of the triplet states in the relaxation dynamics of disulfides. This theoretical work provides insights into the intrinsic excited-state properties of disulfide molecules.
- This article is part of the themed collection: 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...