On the kinetics of the removal of ligands from films of colloidal nanocrystals by plasmas†
Abstract
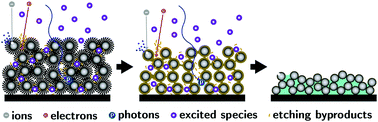
This paper describes the kinetic limitations of etching ligands from colloidal nanocrystal assemblies (CNAs) by plasma processing. We measured the etching kinetics of ligands from a CNA model system (spherical ZrO2 nanocrystals, 2.5–3.5 nm diameter, capped with trioctylphosphine oxide) with inductively coupled plasmas (He and O2 feed gases, powers ranging from 7 to 30 W, at pressures ranging from 100 to 2000 mTorr and exposure times ranging between 6 and 168 h). The etching rate slows down by about one order of magnitude in the first minutes of etching, after which the rate of carbon removal becomes proportional to the third power of the carbon concentration in the CNA. Pressure oscillations in the plasma chamber significantly accelerate the overall rate of etching. These results indicate that the rate of etching is mostly affected by two main factors: (i) the crosslinking of the ligands in the first stage of plasma exposure, and (ii) the formation of a boundary layer at the surface of the CNA. Optimized conditions of plasma processing allow for a 60-fold improvement in etching rates compared to the previous state of the art and make the timeframes of plasma processing comparable to those of calcination.


 Please wait while we load your content...
Please wait while we load your content...