Fast beam photofragment translational spectroscopy of the phenoxy radical at 225 nm, 290 nm, and 533 nm†
Abstract
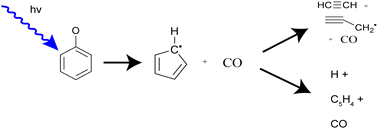
Photodissociation of the phenoxy radical (C6H5O) is investigated using fast beam photofragment translational spectroscopy. Phenoxy radicals are generated through photodetachment of phenoxide anions (C6H5O−) at 532 nm. Following photoexcitation of the radicals at 225 nm (5.51 eV), 290 nm (4.27 eV), or 533 nm (2.33 eV), photofragments are collected in coincidence to determine their masses, translational energy, and scattering angle for each dissociation event. Two-body dissociation yields exclusively CO + C5H5, and three-body dissociation to CO + C2H2 + C3H3 and CO + C5H4 + H is also seen at the two higher energies. The translational energy distributions for two-body dissociation suggest that dissociation occurs via internal conversion to the ground electronic state followed by statistical dissociation. The absorption of an additional 532 nm photon in the photodetachment region provides some C6H5O radicals with an additional 2.33 eV of energy, leading to much of the two-body dissociation observed at 533 nm and the three-body dissociation at the two higher excitation energies.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...