Mapping the intrinsic absorption properties and photodegradation pathways of the protonated and deprotonated forms of the sunscreen oxybenzone†
Abstract
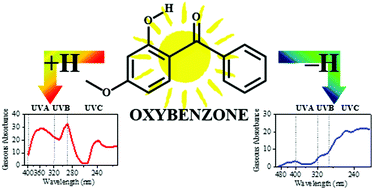
Sunscreens provide vital protection against the photodamaging effects of UV radiation, however, many fundamental questions remain about the detailed mechanisms by which they dissipate UV energy. One such issue is the extent to which the pH environment of an organic sunscreen molecule alters its effectiveness, both in terms of ability to absorb UV radiation, and also its potential to photodegrade. Here, we use gas-phase laser photodissociation spectroscopy for the first time to measure the intrinsic UVA–UVC absorption spectra and associated photodegradation products of protonated and deprotonated oxybenzone, away from the complications of bulk mixtures. Our results reveal that protonation state has a dramatic effect on the absorption and photodissociation properties of this sunscreen. While the UV absorption profile of oxybenzone is only modestly affected by protonation across the range from 400–216 nm, deprotonated oxybenzone displays a significantly modified absorption spectrum, with very low photoabsorption between 370–330 nm. Protonated oxybenzone primarily photofragments by rupture of the bonds on either side of the central carbonyl group, producing cationic fragments with m/z 151 and 105. Additional lower mass photofragments (e.g. m/z 95 and 77) are also observed. The production spectra for the photofragments from protonated oxybenzone fall into two distinct categories, which we discuss in the context of different excited state decay pathways. For deprotonated oxybenzone, the major photofragments observed are m/z 211 and 212, which are associated with the ejection of methane and the methyl free radical from the parent ion, respectively. Implications for the suitability of oxybenzone in its protonated and deprotonated forms as an optimum sunscreen molecule are discussed.
- This article is part of the themed collections: PCCP Editor’s Choice, 2020 and Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...