A DFT study of direct furfural conversion to 2-methylfuran on the Ru/Co3O4 surface†
Abstract
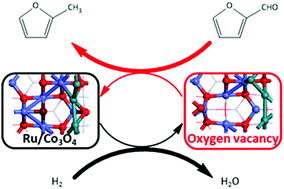
Furfural conversion via hydrodeoxygenation pathways on the Ru/Co3O4 surface is thoroughly investigated using density functional theory calculations. All feasible steps are identified. It is found that an oxygen vacancy is necessary to be generated in the form of water for the subsequent hydrodeoxygenation of furfural. The furfural adsorbs at oxygen vacancy sites in an η2(C–O) pattern. The hydrodeoxygenation product, 2-methylfuran, is yielded via the hydrogenation of furfural into furyl–CH2O alkoxide intermediate, followed by C–O cleavage, and finally the hydrogenation of the unsaturated furyl–CH2 species. This reaction pathway is both kinetically and thermodynamically facile. The by-product furfuryl alcohol could be attributed to the outstanding hydrogenation ability of the ruthenium metal. Comparing to the group X metals and ruthenium, the decarbonylation pathway to produce furan and carbon monoxide is inhibited by the adsorption geometry.


 Please wait while we load your content...
Please wait while we load your content...