The role of symmetric functionalisation on photoisomerisation of a UV commercial chemical filter†
Abstract
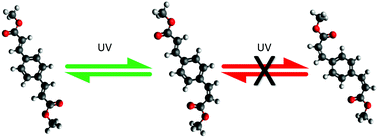
Photoisomerisation has been shown to be an efficient excited-state relaxation mechanism for a variety of nature-based and artificial-based molecular systems. Here we report on the excited-state relaxation dynamics and consequent photostability of a symmetrically functionalised cinnamate by transient electronic absorption spectroscopy, along with complementary computational and steady-state spectroscopy methods. The findings are then discussed in comparison to 2-ethylhexyl-E-4-methoxycinnamate, a structurally related ‘off the shelf’ chemical filter present in commercial sunscreens with a similar absorption profile. The present study allows for a like-for-like comparison beween 2-ethylhexyl-E-4-methoxycinnamate and the functionalised cinnamate, driven by the need to enhance solar protection across both the UVA and UVB regions of the electromagnetic spectrum.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...