Crystallization kinetics of thin water films on Pt(111): effects of oxygen and carbon-monoxide adspecies
Abstract
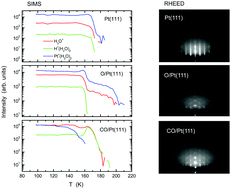
This paper describes nucleation, epitaxial growth, and wettability of water on Pt(111) and how they are influenced by oxygen and carbon-monoxide adspecies, based on reflection high energy electron diffraction (RHEED), time-of-flight secondary ion mass spectrometry (TOF-SIMS), and temperature-programmed desorption (TPD). Amorphous solid water deposited onto the pristine Pt(111) substrate crystallizes into ice Ih together with a 2D layer at 150 K, whereas ice Ic (stacking disordered ice or a mixture of ice Ic and Ih) is formed preferentially onto oxygenated Pt(111) (CO-adsorbed Pt(111)) at 155–160 K (150 K). The ice nucleation and epitaxial growth tend to be hampered on the oxygenated Pt(111) surface via hydrogen bond formation with chemisorbed oxygen. The CO-adsorbed Pt(111) surface is hydrophobic, as evidenced by the fact that water forms a complex with CO during evaporation of crystallites at 160–165 K. A disordered 2D layer remains on pristine Pt(111) up to 175 K, whereas an ordered 2D layer exhibiting the (√3 × √3)R30° structure formed on oxygenated Pt(111) up to 200 K.
- This article is part of the themed collections: 2019 PCCP HOT Articles and 2018 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...