Mechanistic study of the reaction of CH2F2 with Cl atoms in the absence and presence of CH4 or C2H6: decomposition of CHF2OH and fate of the CHF2O radical†
Abstract
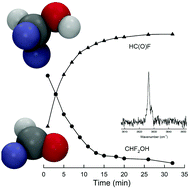
To assess the atmospheric fate of fluorinated compounds, chamber experiments were performed with Fourier transform infrared spectroscopy investigating the products of difluoromethane, CH2F2, at 296 ± 2 K. The reactions were initiated by reaction of CH2F2 with Cl atoms in the absence and presence of CH4 or C2H6 in air or O2. No evidence of formation of the fluorinated alcohol, CHF2OH, from the reactions of the CHF2O2 radical with either CH3O2 or CH3CH2O2 was observed. However, evidence of an alkoxy radical pathway was observed to form CHF2OH. The alkoxy radical, CHF2O, abstracts a hydrogen atom from CH2F2 (with reaction mixtures of high initial CH2F2 concentrations) to give the alcohol CHF2OH that in turn decomposes with a rate coefficient of k(CHF2OH) = (1.68 × 10−3 ± 0.19 × 10−3) s−1, giving a half-life of the alcohol of (412 ± 48) s. Theoretical calculations indicate that the CHF2OH decomposition is unlikely to be a unimolecular process, and we instead propose that it is catalyzed by –OH groups present in molecules, or on particles or surfaces. HC(O)F is formed in a yield indistinguishable from 100% from the decomposition of CHF2OH. The competition between the reaction of CHF2O radicals with O2 and with CH2F2 was investigated and an experimental rate coefficient ratio of 0.57 ± 0.08 of reaction with O2 over reaction with CH2F2 was determined. Ab initio calculations support a larger reaction barrier for the O2 reaction by 0.5 kcal mol−1, with transition state theory predicting a rate coefficient ratio of 0.35, in reasonable agreement with experiment. The primary product of the atmospheric degradation of CH2F2 is expected to be C(O)F2 formed by the reaction of CHF2O with O2.


 Please wait while we load your content...
Please wait while we load your content...