Dynamics and quantum yields of H2 + CH2CO as a primary photolysis channel in CH3CHO†
Abstract
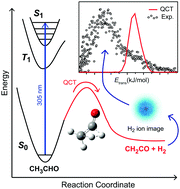
The first experimental observation of the primary photochemical channel of acetaldehyde leading to the formation of ketene (CH2CO) and hydrogen (H2) molecular products is reported. Acetaldehyde (CH3CHO) was photolysed in a molecular beam at 305.6 nm and the resulting H2 product characterized using velocity-map ion (VMI) imaging. Resonance-enhanced multiphoton ionization (REMPI), via two-photon excitation to the double-well EF 1Σ+g state, was used to state-selectively ionize the H2 and determine angular momentum distributions for H2 (ν = 0) and H2 (ν = 1). Velocity-map ion images were obtained for H2 (ν = 0 and 1, J = 5), allowing the total translational energy release of the photodissociation process to be determined. Following photolysis of CH3CHO in a gas cell, the CH2CO co-fragment was identified, using Fourier transform infrared spectroscopy, by its characteristic infrared absorption at 2150 cm−1. The measured quantum yield of the CH2CO + H2 product channel at 305.0 nm is ϕ = 0.0075 ± 0.0025 for both 15 Torr of neat CH3CHO and a mixture with 745 Torr of N2. Although small, this result has implications for the atmospheric photochemistry of carbonyls and this reaction represents a new tropospheric source of H2. Quasi-classical trajectory (QCT) simulations on a zero-point energy corrected reaction-path potential are also performed. The experimental REMPI and VMI image distributions are not consistent with the QCT simulations, indicating a non reaction-path mechanism should be considered.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...