K–O2 electrochemistry: achieving highly reversible peroxide formation†
Abstract
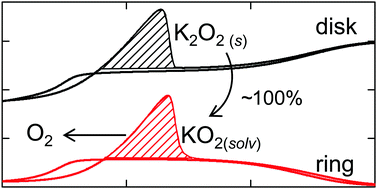
Since the advent of the lithium–air battery, researchers have focused on understanding the underpinning mechanisms of the oxygen reduction and evolution reaction in aprotic solvents. In this work, the oxygen reaction in the presence of potassium ions in dimethyl sulfoxide was exploited as a model system to refine the present mechanistic picture of oxygen reduction in aprotic environments. In a combined approach utilizing differential electrochemical mass spectrometry in a generator–collector arrangement as well as classical electrochemical techniques, the reversible formation of insoluble peroxide as well as of slightly soluble superoxide is shown. As opposed to other peroxides in other non-aqueous metal–oxygen systems, potassium peroxide can be reoxidized to superoxide with an overpotential of as little as 100 mV. The investigation of the effect of the oxygen partial pressure between 0 and 1 atmosphere demonstrates how the precipitation of superoxide increases the oxidation overpotential of the peroxide and establishes a link between this work and other studies, in which the reversibility of the peroxide formation has not been identified.


 Please wait while we load your content...
Please wait while we load your content...