Effects of the long octyl chain on complex formation of nickel(ii) with dimethyl sulfoxide, methanol, and acetonitrile in ionic liquid of [C8mim][TFSA]†
Abstract
In the room-temperature ionic liquid (IL) of 1-methyl-3-octylimidazolium bis(trifluoromethylsulfonyl)amide ([C8mim][TFSA]), the complex formation of Ni2+ with molecular liquids (MLs), dimethyl sulfoxide (DMSO), methanol (MeOH), and acetonitrile (AN), has been examined using ultraviolet (UV)-visible spectroscopy. The overall stability constants log βn, enthalpies 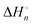 , and entropies
, and entropies 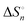 of the equilibria have been determined to elucidate the mechanism of complex formation. From a comparison of such thermodynamic parameters of the present [C8mim][TFSA] systems with those of the previous systems of 1-ethyl-3-methylimidazolium-based IL, [C2mim][TFSA], the effects of the octyl chain of the imidazolium cation, [C8mim]+, on the complex formation of Ni2+ with MLs have been demonstrated. In [C8mim][TFSA]–ML systems, more stable complexes are formed with MLs in the sequence of AN > DMSO ≫ MeOH. This sequence differs from that of DMSO ≫ AN > MeOH in [C2mim][TFSA]. For the AN systems, the stabilities of [Ni(an)n] in [C8mim][TFSA] are higher as compared to those in [C2mim][TFSA]. In contrast, for the DMSO systems, [Ni(dmso)n] is less stable in the IL with the longer alkyl chain than that in the IL with the shorter chain. The dependence of the alkyl chain length on the stabilities of [Ni(meoh)n] is the least significant among the three MLs. These varieties of the stabilities of Ni2+ complexes with the MLs have been interpreted from the thermodynamic parameters, together with the static interactions in the [C8mim][TFSA]–ML and [C2mim][TFSA]–ML solvents observed by means of 1H and 13C NMR, small-angle neutron scattering (SANS), and infrared (IR) with an ATR diamond prism.
of the equilibria have been determined to elucidate the mechanism of complex formation. From a comparison of such thermodynamic parameters of the present [C8mim][TFSA] systems with those of the previous systems of 1-ethyl-3-methylimidazolium-based IL, [C2mim][TFSA], the effects of the octyl chain of the imidazolium cation, [C8mim]+, on the complex formation of Ni2+ with MLs have been demonstrated. In [C8mim][TFSA]–ML systems, more stable complexes are formed with MLs in the sequence of AN > DMSO ≫ MeOH. This sequence differs from that of DMSO ≫ AN > MeOH in [C2mim][TFSA]. For the AN systems, the stabilities of [Ni(an)n] in [C8mim][TFSA] are higher as compared to those in [C2mim][TFSA]. In contrast, for the DMSO systems, [Ni(dmso)n] is less stable in the IL with the longer alkyl chain than that in the IL with the shorter chain. The dependence of the alkyl chain length on the stabilities of [Ni(meoh)n] is the least significant among the three MLs. These varieties of the stabilities of Ni2+ complexes with the MLs have been interpreted from the thermodynamic parameters, together with the static interactions in the [C8mim][TFSA]–ML and [C2mim][TFSA]–ML solvents observed by means of 1H and 13C NMR, small-angle neutron scattering (SANS), and infrared (IR) with an ATR diamond prism.
![Graphical abstract: Effects of the long octyl chain on complex formation of nickel(ii) with dimethyl sulfoxide, methanol, and acetonitrile in ionic liquid of [C8mim][TFSA]](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CP06345A&imageInfo.ImageIdentifier.Year=2019)


 Please wait while we load your content...
Please wait while we load your content...