Pronounced changes in atomistic mechanisms for the Cl− + CH3I SN2 reaction with increasing collision energy†
Abstract
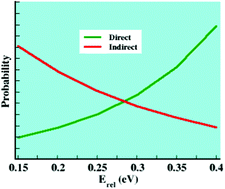
In a previous direct dynamics simulation of the Cl− + CH3I → ClCH3 + I− SN2 reaction, predominantly indirect and direct reaction was found at collision energies Erel of 0.20 and 0.39 eV, respectively. For the work presented here, these simulations were extended by studying the reaction dynamics from Erel of 0.15 to 0.40 eV in 0.05 eV intervals. A transition from a predominantly indirect to direct reaction is found for Erel of 0.27–0.28 eV, a finding consistent with experiment. The simulation results corroborate the understanding that in experiments indirect reaction is characterized by small product translational energies and isotropic scattering, while direct reaction has higher translational energies and anisotropic scattering. The traditional statistical theoretical model for the Cl− + CH3I SN2 reaction assumes the Cl−–CH3I pre-reaction complex (A) is formed, followed by barrier crossing, and then formation of the ClCH3–I− post-reaction complex (B). This mechanism is seen in the dynamics, but the complete atomistic dynamics are much more complex. Atomistic SN2 mechanisms contain A and B, but other dynamical events consisting of barrier recrossing (br) and the roundabout (Ra), in which the CH3-moiety rotates around the heavy I-atom, are also observed. The two most important mechanisms are only formation of A and Ra + A. The simulation results are compared with simulations and experiments for Cl− + CH3Cl, Cl− + CH3Br, F− + CH3I, and OH− + CH3I.


 Please wait while we load your content...
Please wait while we load your content...