Symmetric dissociation of the water molecule with truncation energy error. A benchmark study†
Abstract
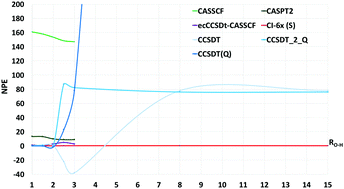
We use selected configuration interaction with truncation energy error (SCI-TEE) and CI by parts (CIBP) to study the symmetric dissociation of the water molecule with Roos' triple-ζ double polarization basis set and with the Dunning cc-pVTZ basis. The calculations comprise CISDTQ (CI-4x) through CI-8x for H2O at its equilibrium geometry (Req) and up to fifteen times Req. With the Dunning basis our SCI-TEE-8x energies differ from full CI by less than 0.01 mHartree (0.006 kcal mol−1) at all O–H distances, representing the best upper bounds for this system outside Req. We compare our results with those of other relevant ab initio methods finding good agreement with recent DMRG calculations. The non-parallelity error (NPE) for SCI-TEE-6x remains stable below 0.1 mHartree when moving from the Roos to the Dunning orbitals. For the present system, CBS energy errors at the experimental equilibrium geometry and at dissociation can accurately be evaluated as the difference between non-relativistic total electronic energies taken from the literature, and our SCI-TEE-8x energies obtained with Dunning's or Roos' orbitals. In both cases, the difference between CBS energy errors at the equilibrium geometry and dissociation is not smaller than 10 mH, showing that chemically accurate NPE values do not guarantee a chemically accurate potential energy surface.
- This article is part of the themed collections: Celebrating Latin American Talent in Chemistry and Celebrating recent chemical science in Mexico


 Please wait while we load your content...
Please wait while we load your content...