Graphene–hBN non-van der Waals vertical heterostructures for four- electron oxygen reduction reaction†
Abstract
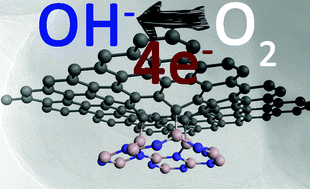
A novel vertical non-van der Waals (non-vdW) heterostructure of graphene and hexagonal boron nitride (G/hBN) is realized and its application in direct four-electron oxygen reduction reaction (ORR) in alkaline medium is established. The G/hBN differs from previously demonstrated vdW heterostructures, where it has a chemical bridging between graphene and hBN allowing a direct charge transfer – resulting in high ORR activity. The ORR efficacy of G/hBN is compared with that of graphene–hBN vdW structure and individual layers of graphene and hBN along with that of benchmark platinum/carbon (Pt/C). The ORR activity of G/hBN is found to be on par with Pt/C in terms of current density but with much higher electrochemical stability and methanol tolerance. The onset potential of the G/hBN is found to be improved from 780 mV at a glassy carbon electrode to 930 mV and 940 mV in gold and platinum electrodes, respectively, indicating its substrate-dependent catalytic activity. This opens possibilities of new benchmark catalysts of metals capped with G/hBN atomic layers, where the underneath metal is protected while keeping the activity similar to that of pristine metal. Density functional theory-based calculations are found to be supporting the observed augmented ORR performance of G/hBN.


 Please wait while we load your content...
Please wait while we load your content...