Hydrogen adsorption trends on various metal-doped Ni2P surfaces for optimal catalyst design†
Abstract
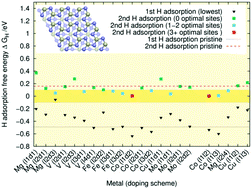
In this study, we looked at the hydrogen evolution reaction on Mg-, Mo-, Fe-, Co-, V-, and Cu-doped Ni3P2 and Ni3P2 + P terminated Ni2P surfaces. The DFT calculated hydrogen adsorption free energy was employed as a predictor of the materials' catalytic HER activity. Our results indicate that doping can substantially improve the catalytic activity of the Ni3P2 terminated surface. In contrast, the Ni3P2 + P terminated one seems to be catalytically active irrespective of the type of doping, including in the absence of doping. Based on our doping energy and adsorption free energy calculations, the most promising dopants are iron and cobalt, whereas copper is less likely to function well as a doping element.


 Please wait while we load your content...
Please wait while we load your content...