Tuning polaronic redox behavior in olivine phosphate†
Abstract
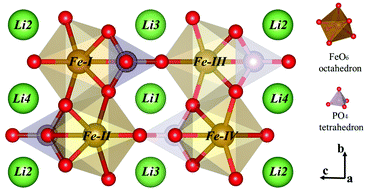
In order to understand and improve the conductivity of LiFePO4, lots of attempts have been made both experimentally and theoretically. Here we performed hybrid density functional theory calculations to systematically investigate the electronic structures with polaronic redox behavior of polyanionic intercalation compounds similar to LiFePO4, such as in XMPO4 (X = Li, Na; M = Mn, Fe, Co, Ni). It is proved that the replacement of Li ions does not eliminate the polaronic redox behavior of Fe ions during delithiation and hence does not lead to a significant improvement in electronic conductivity. By contrast, replacing Fe with Mn, Co or Ni can tune the polaronic redox behavior during delithiation by varying degrees. For Ni, the polaronic redox behavior has almost disappeared, and band gaps disappear during delithiation, indicating a better electronic conductivity. For Mn or Co, the polaronic redox behavior is still obvious with little improvement in the electronic conductivity. This study provides important clues to improve the electronic conductivity of LiFePO4-like cathode materials.


 Please wait while we load your content...
Please wait while we load your content...