Theoretical study on the reaction mechanism and selectivity of acetylene semi-hydrogenation on Ni–Sn intermetallic catalysts†
Abstract
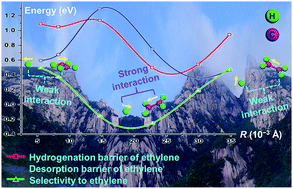
Recently, Ni–Sn intermetallic compounds (IMCs) with unique geometric structures have been proved to be selective catalysts for acetylene hydrogenation to ethylene, but the origin of the selectivity remains unclear. In this work, a density functional theory (DFT) study has been carried out to investigate the mechanism of acetylene hydrogenation on six surfaces of Ni–Sn IMCs, and the geometric effects towards ethylene selectivity were revealed. Two key parameters (adsorption energy and the hydrogenation barrier of ethylene), which determine the ethylene selectivity, were studied quantitatively. The adsorption sites for C2Hy (y = 2, 3, 4) can be classified into three types: Type 1 (Ni3Sn(111) and Ni3Sn2(101)-2) with Ni trimers, Type 2 (Ni3Sn(001) and Ni3Sn2(001)) with Ni monomers, and Type 3 (Ni3Sn2(101) and Ni3Sn2(001)-2) with reconstructed metal trimers. The adsorption energy (Ead) decreases following the order: Type 1 > Type 3 > Type 2, which indicates that the adsorption strength depends significantly on site ensemble: a more isolated Ni site would facilitate the desorption of ethylene. However, the surface roughness mainly dominates the hydrogenation barrier of ethylene. Either low or high roughness decreases the interactions between H and C2H4 (Eint), resulting in an enhanced energy barrier for over-hydrogenation of C2H4 (Ea,hydr); while moderate roughness benefits Eint and lowers Ea,hydr. The selectivity to ethylene is denoted as ΔEa = Ea,hydr − |Ead|, thus depending on the interplay of site ensemble effects and surface roughness. From this point of view, Ni3Sn(001) and Ni3Sn2(101) surfaces with well-isolated Ni ensembles and low (or high) surface roughness exhibit decreased |Ead| and increased Ea,hydr, giving rise to excellent selectivity to ethylene. This work provides significant understanding of the origin of ethylene selectivity in terms of geometric effects, which gives helpful instruction for the design and preparation of intermetallic catalysts for acetylene semi-hydrogenation.


 Please wait while we load your content...
Please wait while we load your content...