Highly localized H2O librational motion as a far-infrared spectroscopic probe for microsolvation of organic molecules
Abstract
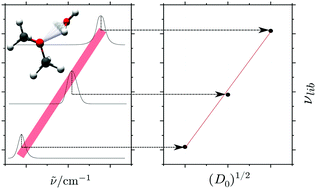
The most prominent spectroscopic observable for the hydrogen bonding between individual molecules in liquid water is the broad absorption band detected in the spectral region between 300 and 900 cm−1. The present work demonstrates how the associated large-amplitude out-of-plane OH librational motion of H2O molecules also directly reflects the microsolvation of organic compounds. This highly localized OH librational motion of the first solvating H2O molecule causes a significant change of dipole moment and gives rise to a strong characteristic band in the far-infrared spectral region, which is correlated quantitatively with the complexation energy. The out-of-plane OH librational band origins ranging from 324.5 to 658.9 cm−1 have been assigned experimentally for a series of four binary hydrogen-bonded H2O complexes embedded in solid neon involving S-, O- and N-containing compounds with increasing hydrogen bond acceptor capability. The hydrogen bond energies for altogether eight binary H2O complexes relative to the experimental value of 13.2 ± 0.12 kJ mol−1 for the prototypical (H2O)2 system [Rocher-Casterline et al., J. Chem. Phys., 2011, 134, 211101] are revealed directly by these far-infrared spectroscopic observables. The far-infrared spectral signatures are able to capture even minor differences in the hydrogen bond acceptor capability of O atoms with slightly different alkyl substituents in the order H–O–C(CH3)3 > CH3–O–CH3 > H–O–CH(CH3)2 > H–O–CH2CH3.


 Please wait while we load your content...
Please wait while we load your content...