Proton transfer from pinene stabilizes water clusters†
Abstract
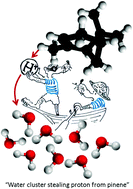
We ionize small mixed pinene–water clusters by electron impact or by using photons after sodium doping and analyze the products by mass spectrometry. Electron ionization results in the formation of pure pinene, mixed pinene–water and protonated water cluster cations. The “fragmentation free” photoionization after sodium doping results into the formation of only water–Na+ clusters with a mean cluster size below that observed after electron ionization. We show that protonated water clusters are formed both directly and indirectly via pinene ionziation. The latter pathway is detailed by ab intio calculations, demonstrating the feasibility of proton transfer from pinene for larger water clusters. In small clusters, the proton transfer reaction is controlled by proton solvation energy and we can thus estimate its value for finite size clusters. The observed stabilization mechanism of water clusters may contribute to the formation of cloud condensation nuclei in the atmosphere.
- This article is part of the themed collection: Photodissociation and reaction dynamics


 Please wait while we load your content...
Please wait while we load your content...