Sensitivity analysis of the variability of amyloid aggregation profiles†
Abstract
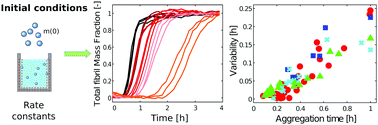
In vitro kinetic assays of amyloid formation represent a central tool in many areas of biotechnological and biomedical sciences, including drug discovery against a variety of neurodegenerative disorders. The conversion of soluble peptides and proteins into insoluble amyloid aggregates follows macroscopic kinetic profiles that are commonly characterized by a certain degree of variability. This variability can challenge the investigation of the molecular determinants of the aggregation process, and its molecular origin remains largely elusive. Here, we analyze the aggregation profiles of four different amyloidogenic proteins that follow distinct microscopic mechanisms. We show that the variability of the kinetic traces, described by the standard deviation of the aggregation lag-phase, is linearly proportional to the duration of the aggregation process. By applying a sensitivity analysis we demonstrate that this behaviour arises from an initial fixed perturbation of one or more of the kinetic parameters of the aggregation network, and does not involve any amplification of the perturbation during the aggregation process. Moreover, our parametric sensitivity analysis shows that the experimentally measured variability is compatible with variations in the initial monomer concentration that are consistently smaller compared to all the other kinetic parameters, denoting a higher sensitivity of the amyloid aggregation process with respect to this parameter. Overall, these results show that the variability of aggregation profiles in large volumes (μl) depends on the initial conditions and not on intrinsic stochasticity. The accurate control of the initial conditions is therefore crucial to decrease this variability.


 Please wait while we load your content...
Please wait while we load your content...