Elucidating cation–cation interactions in neptunyl dications using multi-reference ab initio theory†
Abstract
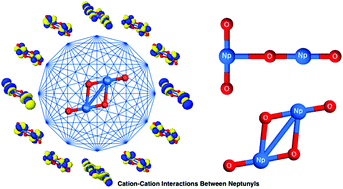
Understanding the binding mechanism in neptunyl clusters formed due to cation–cation interactions is of crucial importance in nuclear waste reprocessing and related areas of research. Since experimental manipulations with such species are often rather limited, we have to rely on quantum-chemical predictions of their electronic structures and spectroscopic parameters. In this work, we present a state-of-the-art quantum chemical study of the T-shaped and diamond-shaped neptunyl(V) and neptunyl(VI) dimers. Specifically, we scrutinize their molecular structures, (implicit and explicit) solvation effects, the interplay of static and dynamical correlation, and the influence of spin–orbit coupling on the ground state and lowest-lying excited states for different total spin states and total charges of the neptunyl dications. Furthermore, we use the picture of interacting orbitals (quantum entanglement and correlation analysis) to identify strongly correlated orbitals in the cation–cation complexes that should be included in complete active space calculations. Most importantly, our study highlights the complex interplay of correlation effects and relativistic corrections in the description of the ground and lowest-lying excited states of neptunyl dications.


 Please wait while we load your content...
Please wait while we load your content...