Abstract
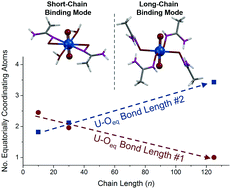
Emergence is complex behavior arising from the interactions of many simple constituents that do not display such behavior independently. Polyamidoxime (PAO) uranium adsorbents show such phenomena, as recent works articulate that the polymer binds uranium differently than the monomeric constituents. In order to investigate the origins of this emergent uranium-binding behavior, we synthesized a series of amidoxime polymers with low polydispersity and small molecules with lengths ranging from 1 to 125 repeat units. Following immersion in a uranyl-containing solution, the local, intermediate, and macroscopic structures were investigated by X-ray absorption fine structure (XAFS) spectroscopy, small angle neutron scattering (SANS), and dynamic light scattering (DLS). Fits of the extended XAFS (EXAFS) region revealed a progressive change in uranium coordination environment as a function of polymer molecular weight, identifying chain length as a driving force in emergent metal binding and resolving the controversy over how amidoxime adsorbents bind uranium.


 Please wait while we load your content...
Please wait while we load your content...
