Interpenetration isomers in isoreticular amine-tagged zinc MOFs†
Abstract
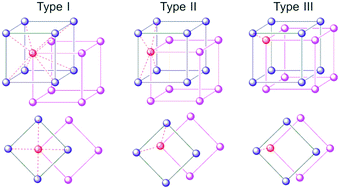
The effect of increasing steric size of pendant amine substituents on structural isoreticulation has been studied systematically in a series of Zn-MOFs. Linear biphenyl dicarboxylic acids tagged with pendant primary amine (H2bpdc-NH2), allylamine (H2bpdc-NHallyl), diallylamine (H2bpdc-N(allyl)2) and dimethylamine (H2bpdc-NMe2) groups react with zinc nitrate in DMF to yield a set of interpenetrated MOFs, WUF-11–14, respectively, that are structurally akin to IRMOF-9. The allylated amine ligands undergo C–N cleavage reactions under the synthesis conditions, yielding WUF-12 and WUF-13 as multivariate MOFs. The single crystal X-ray crystallography on this set of MOFs was not straightforward and we give a salutary account of the difficulties encountered. Gas adsorption measurements combined with surface area calculations provide invaluable support for the crystallographic assignments. The crystallographic analyses reveal subtle differences in the relative positions of the interpenetrating frameworks, and we present a classification system for this type of MOF and analyse related examples available in the literature. CO2 adsorption measurements revealed that WUF-14, which features the strongest Brønsted basic dimethylamine tag group, has the highest capacity, isosteric heat of adsorption, and CO2/N2 selectivity.


 Please wait while we load your content...
Please wait while we load your content...