Ionic liquid-mediated low-temperature formation of hexagonal titanium-oxyhydroxyfluoride particles†
Abstract
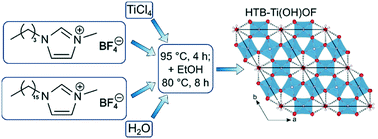
In this work a facile ionic liquid-based synthesis towards titanium oxyhydroxyfluoride Ti(OH)OF is presented. The synthesis is carried out as a one-pot synthesis at a relatively low temperature (95 °C). The ionic liquids are used as both the reaction medium and fluorination source. Powder X-ray diffraction (PXRD) followed by Rietveld refinement revealed a hexagonal crystal structure similar to the hexagonal tungsten bronze (HTB) structure known in the literature. We also show that water is present inside the channels of the crystal structure. Additionally, we performed transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS) and thermogravimetric analysis coupled with mass spectrometry (TGA-MS) measurements. The results of the different methods are in agreement with the HTB-Ti(OH)OF structure. In addition to the structural analysis, the electrochemical performance of the material was studied by measuring galvanostatic charge/discharge profiles and cyclic voltammetry using Li+. We found a rather high specific capacity in the range above 200 mA h g−1 (at 0.1 A g−1) and an excellent rate capability (more than 110 mA h g−1 at 5 A g−1), which makes this material a promising material for future electrochemical applications (e.g. batteries).
- This article is part of the themed collection: Crystal engineering for electrochemical applications


 Please wait while we load your content...
Please wait while we load your content...