Stacked aryl groups in P-resolved cyclic phosphonamides as a new conformational constraint†
Abstract
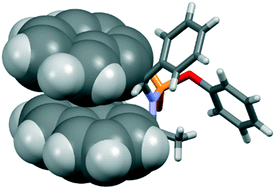
Along the lines of a systematic investigation of conformational constraints promoted by weak interactions, the aryl-stacked structures of some chiral cyclic phosphonamides synthesised with the aid of Betti bases as chirality inducers were investigated by X-ray diffraction analysis and NMR spectroscopy. The synthesised systems showed the stacked conformation between two of their aryl groups, leading to pre-organized structures. The π–π stacking motif between the aromatic rings was observed in solid state and in solution, suggesting that this supramolecular synthon could be used as a conformational constraining tool in the development of drug molecule candidates based on chiral cyclic phosphonamides. The disabling of the π–π stacking motif was pursued by adding an ortho-substituent on one aromatic ring.


 Please wait while we load your content...
Please wait while we load your content...