Selective recognition of Fe3+ and CrO42− ions using a Zn(ii) metallacycle and a Cd(ii) coordination polymer and their heterogeneous catalytic application†
Abstract
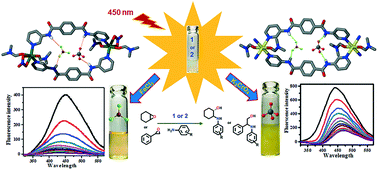
We report the syntheses and structural characterization of a Zn(II) metallacycle [Zn2{(L)2(DMF)2(NO3)2}]·(NO3)2 (1) along with a Cd(II) coordination polymer [{Cd1(L)2(DMF)2}·(NO3)2]n (2) (where L = N,N′-bis-(3-pyridyl)terephthalamide; DMF = dimethylformamide) and their utilization as fluorescent probes for highly selective recognition (turn-off) of Fe3+ and CrO42− ions. Compounds 1 and 2 were prepared by utilizing ligand L and the nitrate salt of zinc and cadmium, respectively. Single crystal X-ray analysis reveals that 1 adopts an overall Zn(II) metallacycle architecture, whereas 2 displays a one-dimensional (1D) polymeric structure. Both 1 and 2 showed high fluorescence stability in aqueous solution and selectively detected Fe3+ and CrO42− ions with high quenching constants and low detection limits of 2.34 × 106 M−1 and 0.153 μM for Fe3+, 6.72 × 105 and 0.205 μM for CrO42− (for 1); 3.25 × 106 and 0.193 μM for Fe3+, 6.94 × 105 and 0.155 μM for CrO42− (for 2). Notably, ligand L itself did not display any type of recognition for any ions in DMF as well as in aqueous media. Moreover, 1 and 2 were also employed as heterogeneous catalysts for the ring-opening reaction (ROR) of epoxides with various assorted amines and up to 95% yield (TON, 47.5 and TOF, 11.9 h−1) was observed with perfect regioselectivity in the case of styrene oxide.


 Please wait while we load your content...
Please wait while we load your content...