CeO2 supported low-loading Au as an enhanced catalyst for low temperature oxidation of carbon monoxide†
Abstract
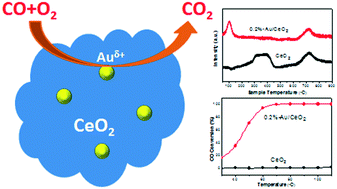
A CeO2-supported Au nanohybrid is regarded as a promising catalytic system for CO oxidation, but its unsatisfactory catalytic activity and high cost are still challenges for sustainable application. In this work, we have synthesized a series of low loading and high activity Au/CeO2 supported catalysts using a coprecipitation method. Au/CeO2 catalysts with a low Au content (0.2 wt%) showed extremely high activity for CO oxidation with 100% conversion of CO around 60 °C. The key to the success is that the unique oxygen-deficient structure of cerium oxide can capture gold to provide a strong synergistic effect between the metal and the support. The content of gold in their oxidation states (Au+1, Au+3) present in the catalyst is one of the important factors affecting the catalytic activity. Furthermore, all of the prepared catalysts present a high BET surface area of roughly 140 m2 g−1. This high specific surface area provides adequate adsorption sites for CO. Moreover, the low loading of Au is critical to reducing the cost of noble metal-based catalysts and making them practical for industrial applications.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...