Natural and synthetic metal oxalates – a topology approach†
Abstract
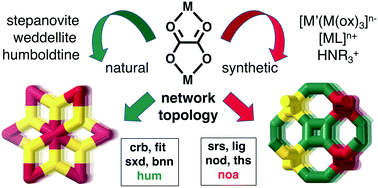
Oxalates are found in minerals and in biology, are made in the laboratory, and are used on an industrial scale. They form coordination polymers and hydrogen bonded networks that often can be analysed using network topology. In this survey of known naturally occurring oxalates we note weddellite, [Ca(C2O4)]·xH2O, that seems to be the first known naturally occurring metal–organic framework, forming the four-connected crb-net (zeolite CRB). The natural oxalates are typically 3D, 2D or 1D coordination polymers, with extensive hydrogen bonding in the latter cases. For example, humboldtine and lindbergite form the new 3- and 8-connected net hum by combining the 1D structure with strong hydrogen bonds. Tris-oxalates rarely occur in nature but stepanovite, [Mg(H2O)6][Na[Fe(ox)3]]·3H2O, is an exception and forms hcb-nets (honeycomb 2D layers) with the hexaaqua ions sealing any potential voids. Synthetic tris-oxalates on the other hand are well explored and normally form 2D hcb-nets or 3D chiral three-connected srs-nets. Theoretically a few other topologies should also be possible, and it was found that [Mn((R)-salmen)(CH3OH)(CH3CN)][MnCr(ox)3]·0.5CH3OH·1.25CH3CN forms the achiral three-connected lig-net, [Fe(2,6-bis(pyrazol-3-yl)pyridine)2][MnCr(ox)3]·2,6-bis(pyrazol-3-yl)pyridine·CH3OH forms the likewise achiral nod-net and [Cu(trans[14]dien)][KCr(ox)3] the ths-net. A new binodal 3-connected net noa (with the derived 3c-,4c- new net mys) was found in [FeII(tren(imid)3)]2[Mn2.5(CH3OH)3Cr3(ox)9]·(CH3OH)4.75·(H2O)4. The more complex [Fe(tren(imid)3)]2[Mn2.5(CH3OH)3Cr3(ox)9] forms a new three-nodal 3-connected daz-net.
- This article is part of the themed collection: Introducing the CrystEngComm Advisory Board and their research


 Please wait while we load your content...
Please wait while we load your content...