A series of flexible bis(pyridyl)bis(tetrazole)-modulated coordination polymers: construction, electrochemical properties, dye adsorption and magnetic properties†
Abstract
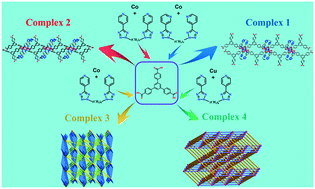
Four new coordination polymers, namely [Co(3-bptzp)(HBTB)]·DMF (1), [Co(4-bptzp)(HBTB)]·EtOH (2), [Co3(4-bptzh)3/2(BTB)2(H2O)] (3), and [Cu4(4-bptzh)2(HBTB)4]·MeCN (4), were prepared by solvothermal reactions of metal(II) salts with 4,4′,4′′-benzene-1,3,5-triyltribenzoic acid (H3BTB) in the presence of different bis(pyridyl)bis(tetrazole) ligands [1,4-bis(5-(3-pyridyl)tetrazolyl)propane (3-bptzp), 1,4-bis(5-(4-pyridyl)tetrazolyl)propane (4-bptzp) and 1,4-bis(5-(4-pyridyl)tetrazolyl)hexane (4-bptzh)] and structurally characterized by single-crystal X-ray diffraction, infrared spectroscopy and powder X-ray diffraction. Complexes 1 and 2 exhibit similar structures, which demonstrate 1D ladder-like chains. Complexes 3 and 4 display different 3D topology frameworks. The diverse architectures of the title complexes show that the spacer length of bis(pyridyl)bis(tetrazole) ligands and metal ions have significant impacts on the structures and the coordination modes of BTB ligands. Dye adsorption and electrochemical and magnetic properties of the title complexes have been investigated, and 3 performed the best among the tested complexes in terms of dye adsorption and electrochemical properties. Complexes 1, 2 and 3 show weak antiferromagnetic behaviors.


 Please wait while we load your content...
Please wait while we load your content...