Formation of supramolecular single and double helix-like structures from designed tripeptides†
Abstract
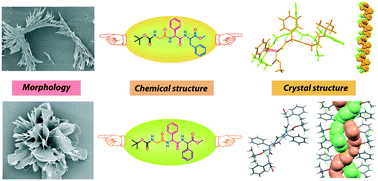
The conformation and self-assembly of N- and C-protected tripeptides, Boc-Gly-L-Phg-D-Phe-OMe (1, Phg: phenylglycine) and Boc-Gly-L-Phg-D-Phg-OMe (2), have been investigated. The effect of the insertion of two non-coded amino acid residues, L-Phg/D-Phe and L-Phg/D-Phg, consecutively on the structure of two tripeptides has been investigated. The single crystal X-ray diffraction analysis of 1 and 2 suggested that both peptides adopted an anti-parallel β-sheet structure but they further self-assembled to form supramolecular single helix and double helix-like architectures, respectively, by various non-covalent interactions in the crystalline state. To the best of our knowledge, this is the first crystallographic report where alternating D/L unnatural amino acid containing small tripeptides exhibited double helix-like architectures. The conformation of these peptides was examined by 2D NMR, solvent dependent NMR titration, and CD spectroscopic studies in solution. Peptides 1 and 2 self-aggregated to form a bunch of flower-petal-like and flower-like architectures, respectively, in an acetonitrile–water medium under a field emission scanning electron microscope (FESEM).


 Please wait while we load your content...
Please wait while we load your content...