Energetic transition metal salts of 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriazole: syntheses, structures and properties†
Abstract
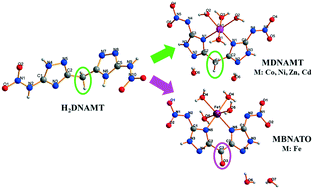
Nitrogen-rich energetic compound 5,5′-dinitramino-3,3′-methylene-1H-1,2,4-bistriazole (H2DNAMT (1)) and its transition metal salts, including [Co(DNAMT)(H2O)4]·2H2O (2), [Ni(DNAMT)(H2O)4]·2H2O (3), [Zn(DNAMT)(H2O)4]·2H2O (4), [Cd(DNAMT)(H2O)4]·2H2O (5) and [Fe(BNATO)(H2O)4]·2H2O (6), were synthesized and characterized by elemental analysis, IR spectroscopy and single-crystal X-ray diffraction analysis. The crystal structures of compounds 2–5 are similar, and crystallize in orthorhombic systems with the Pnma space group, whereas compound 6 belongs to the orthorhombic system with the Pccn space group. The densities of these salts are in the range of 1.832 (compound 2) to 1.999 g cm−3 (compound 5). In compound 6, H2DNAMT was oxidized to bis(3-nitroimino-2H-1,2,4-triazole-5-yl)methanone (H2BNATO) due to the trivalent iron anion. The NBO charge of H2DNAMT and BNATO2− was calculated by density functional theory, to understand their coordination modes. The thermal decomposition processes, non-isothermal kinetics, enthalpies of formation and sensitivities of these compounds were investigated in detail to argue their potential application as energetic materials.


 Please wait while we load your content...
Please wait while we load your content...