Charge density distribution in the crystals of N-n-butyltetrachlorophthalimide. Atoms-in-molecules analysis of different types of halogen interactions†
Abstract
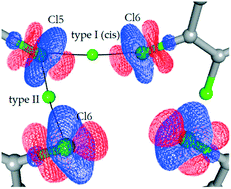
The charge density distribution in N-n-butyltetrachlorophthalimide was experimentally determined using high-resolution X-ray diffraction data and the Hansen–Coppens multipole formalism. These data were used to investigate noncovalent interactions in the supramolecular crystal structure, which were interpreted in terms of Bader's theory of atoms in molecules (AIM). The primary aim of this analysis was to evaluate the chlorine-assisted noncovalent interactions. Topological analysis was performed for C–H⋯Cl hydrogen bonds, C–Cl⋯O halogen bonds and Cl⋯π and Cl⋯Cl contacts. Topological features were used to classify the interactions, which served as a basis for establishing a hierarchy of interactions in the crystal structure. The results demonstrated that the primary forces are short Cl⋯O interactions, which are comparable to moderate hydrogen bonds, while the CH⋯Cl hydrogen bonds and Cl⋯Cl contacts play a secondary role in stabilizing the crystal structure. Based on the topological properties, it is observed that the Cl(lp)⋯π interaction can successfully compete with other intermolecular interactions and thus plays an important role in the observed preference towards molecular stacking, particularly in the absence of strong or moderate hydrogen bonds.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...