Antielectrostatically hydrogen bonded anion dimers: counter-intuitive, common and consistent†
Abstract
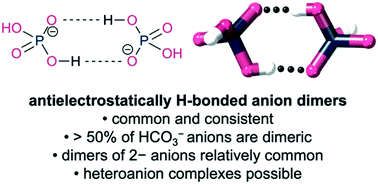
The Cambridge Structural Database was surveyed for antielectrostatically hydrogen bonded anion complexes. The survey revealed that these complexes are relatively common, e.g. half of all structurally characterised bicarbonate anions are present as dimers. Generally, the bonding is surprisingly homogenous with O⋯O contacts close to 2.6 Å for all anions. Distances are similar in length to, or slightly shorter than, those in carboxylic acid dimers, which do not have to contend with Coulombic repulsion. A heteroanion complex was identified as well as a small number of “highly antielectrostatic” dimers formed between two 2− anions.


 Please wait while we load your content...
Please wait while we load your content...