Intramolecular π–hole interactions with nitro aromatics†
Abstract
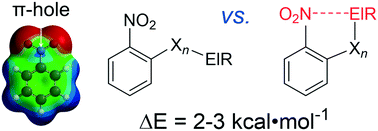
A thorough evaluation of the CSD and DFT computations were conducted to assess if intramolecular π–hole interactions can stabilize a conformer of nitro aromatics. It was found that this can only be the case when the nitro N-atom and an interacting electron-rich atom are separated by at least four bonds. Data from the solid state correspond well to the gas phase calculations and stabilizing energies were estimated to be as large as about 2–3 kcal mol−1, which is in the order of weak hydrogen bonding interactions.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...