Halobenzyl alcohols as structurally simple organogelators†
Abstract
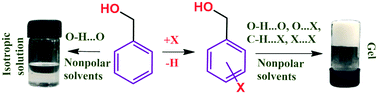
We report 11 simple halobenzyl alcohols, each comprising of only 16 atoms, as organogelators for aliphatic hydrocarbon solvents. Comparison of PXRD profiles of wet gels and xerogels with the simulated profiles from the single crystal XRD data confirmed that the molecular packing in gels and crystals are identical. Therefore single crystal X-ray structures were analyzed to understand the molecular level interactions involved in the self-assembly in gel state. Each of these compounds undergoes a faster linear assembly along the crystallographic direction associated with the OH⋯O hydrogen bond and a supplementary non-covalent interaction (NCI). In directions perpendicular to the direction of OH⋯O Hydrogen bonding, molecules associate through various relatively weaker NCIs. Notably, halogen bonds, X⋯X, C–H⋯X interactions play major role in the lateral growth. In the case of gels, the linear growth is faster than lateral growth leading to the formation of long fibers, which entangles to a 3D fibrous network. This study reveals that halogen atoms in halobenzyl alcohols impart molecular features that contribute to their self-assembly in solution and hence in gelation.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...