Crystal structure and physical stability of ginsenoside compound-K solvates†
Abstract
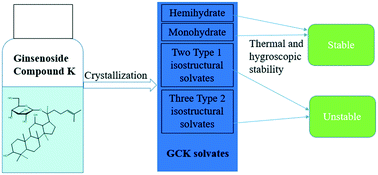
During a comprehensive structure and stability study on the hydrates and alcohol solvates of pharmaceutical molecule ginsenoside compound K (20-O-β-glucopyranosyl-20(S)-protopanaxadiol, GCK), the crystal structure of hemihydrate (HH), ethanol solvate (SEt) and ethanol/water solvate (SEtW) were determined by single-crystal X-ray diffraction (SCXRD) for the first time. All of the solvates were also characterized by powder X-ray diffraction (PXRD), thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). Thermal and hygroscopic stabilities of the solvates were measured by phase transition experiments as well as by stress tests. The results indicate that only the monohydrate exhibited superior physical stability, and the release of solvent molecules made little difference on the crystal architecture for HH, dihydrate (DH) and SEt, while methanol/water solvate (SMeW), SEtW and isopropanol/water solvate (SiPrW) all experienced a phase change during the experiment. Geometry energies among the asymmetric host molecules, hydrogen bond and Hirshfeld analyses, and packing index were used to rationalize the stability of GCK solvates.


 Please wait while we load your content...
Please wait while we load your content...